How Is the Electronegativity Trend Related to the First Ionization
First it tells us the electrons energy level which we often refer to as its shell. NEET Chemistry section is deemed as the scoring section as chemical reactions mechanisms compositions chain reactions for students typically is the bare minimum fundamentals to be covered.
Periodic Trends In Electronegativity Ck 12 Foundation
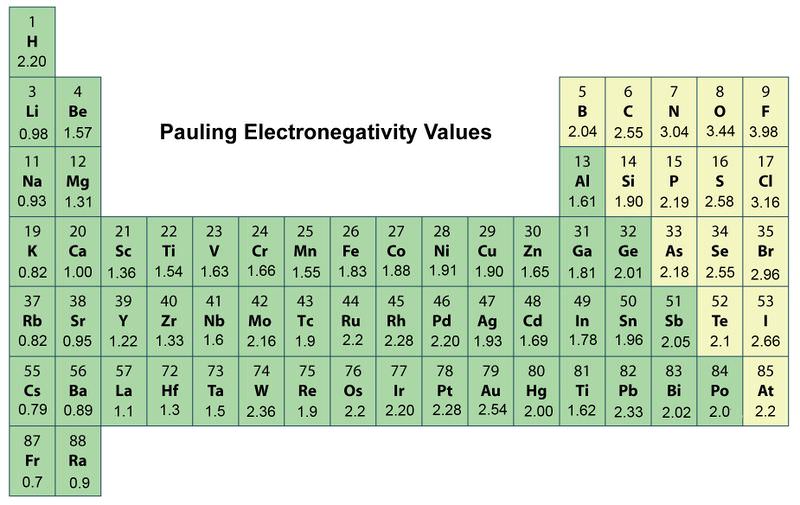
At the same time Cs 5 has electronegativity of 1665 eVindeed ionization or any other involvement of the inner electrons of a Cs atom in chemical bonding is unthinkable at normal conditions.

. Electronegativity symbolized as χ is the tendency for an atom of a given chemical element to attract shared electrons or electron density when forming a chemical bond. Interactive periodic table showing names electrons and oxidation states. The ionization energy trend refers to how the amount of ionization energy needed to remove an.
1 st 2 nd and 3 rd Ionization Energies. Electronegativity also decreases as you move down a group in the table from top to bottom. NEET Chemistry Syllabus is constituted by Physical Chemistry Inorganic Chemistry and Organic Chemistry sections from classes 11 and 12.
Visualize trends 3D orbitals isotopes and mix compounds. Each succeeding ionization energy is larger than the preceding energy. An atoms electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus.
The transition metals have little variance up in them. Second it tells us the orbitals size. Potassium K is an alkali metal placed under sodium and over rubidium and is the first element of period 4.
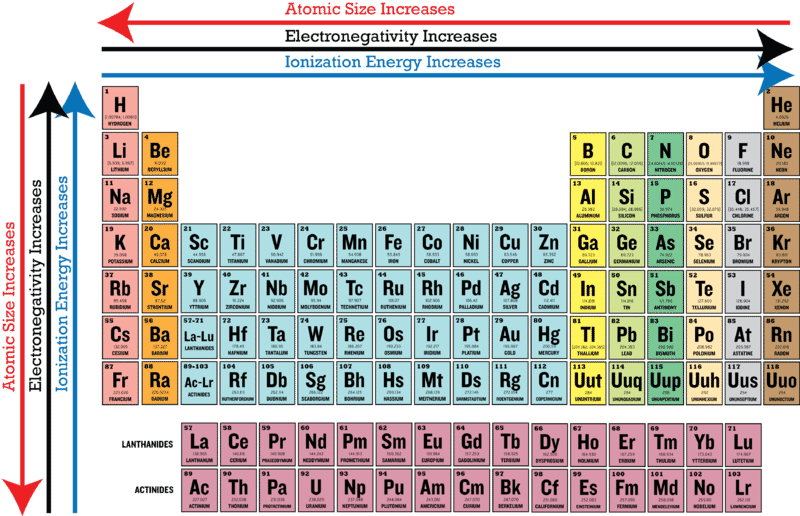
The symbol I_1 stands for the first ionization energy energy required to take away an electron from a neutral atom and the symbol I_2 stands for the second ionization energy energy required to take away an electron from an atom with a 1 charge. On the other hand the properties like Atomic radii ionic radii and metallic character decrease as we move from the left to right direction in the periodic table and increase in the top to bottom. Electronegativity ionization energy and electron affinity increase as we move from the left to right direction in the periodic table and these properties decrease as we move top to bottom.
It is one of the most reactive elements in the periodic table therefore usually only found in compoundsIt tends to oxidize in air very rapidly thus accounting for its rapid reaction with oxygen when freshly exposed to air. Notable exceptions to the electronegativity trend include Ianthanides actinides and noble gases. The situation changes dramatically with pressure and at 200 GPa Cs 5 has the electronegativity of 483 eV lower than that of neutral F 523 eV.
The higher the associated electronegativity the.

What Did You Do Today At School Electronegativity Trends 3d Periodic Table Chemistry Activities Teaching Chemistry High School Science Activities

Welcome To Learnapchemistry Com Teaching Chemistry Ionization Energy Chemistry Classroom

Trends In The Periodic Table Chpt 7 1 Atomic Radius Size 2 Ionization Energy 3 Electronegativity The Ionization Energy Periodic Table Covalent Bonding
Periodic Trends In Electronegativity Ck 12 Foundation

Pin By Katie Rose On My Saves Periodic Table Ionization Energy Chemistry Lessons

Periodic Trends Made Easy Chemtalk

Periodic Trends Chemwiki Electron Affinity Ionization Energy Chemistry Lessons

Easy To Use Chart Of Periodic Table Trends Teaching Chemistry Chemistry Classroom Science Notes

8 4 Ionization Energy Ionization Energy Chemistry Chemistry Experiments

Chart Summarizes The Major Trends In The Properties For Elements In The Periodic Table Trends Electr Chemistry Lessons Chemistry Classroom Teaching Chemistry

Periodic Trends Chemwiki Ionization Energy Chemistry Electron Affinity

File Periodic Trends Svg Wikipedia The Free Encyclopedia Chemistry Classroom Chemistry Chemistry Lessons

Periodic Property Deviations In The Trend Of Ionization Energy Ionization Energy Science Anchor Charts Study Materials

Periodic Table Trends Chemistry Classroom Ionization Energy Middle School Literacy

Ionization Energy Ionization Energy Geometry Worksheets Super Teacher Worksheets

Periodic Table Outermost Electron Orbitals Science Notes And Projects Physical Science Teaching Chemistry Chemistry Classroom

Trends In The Periodic Ta Le Ionization Energy Atomic Radius Electron Affinity Electronegativity Ionization Energy Electron Affinity Electrons

Comments
Post a Comment